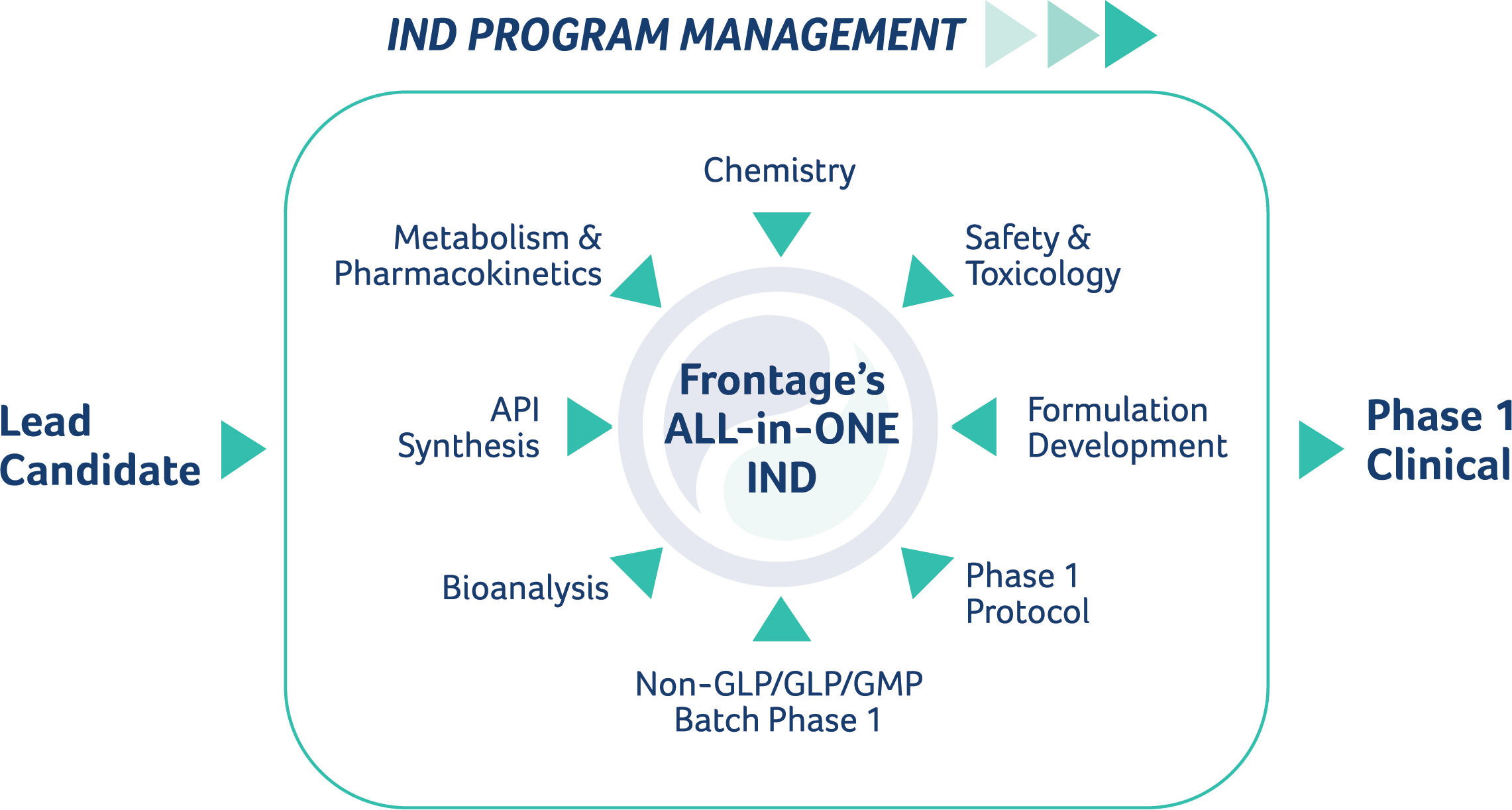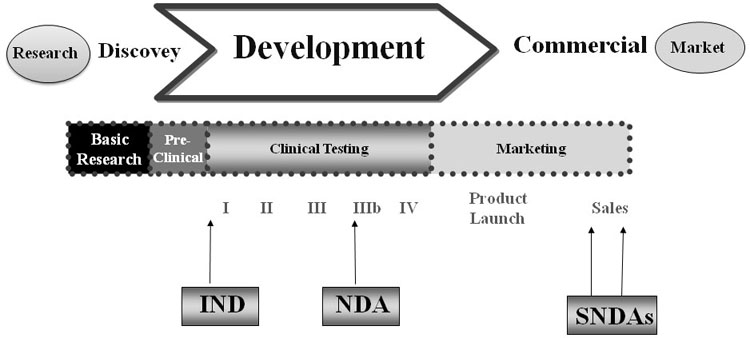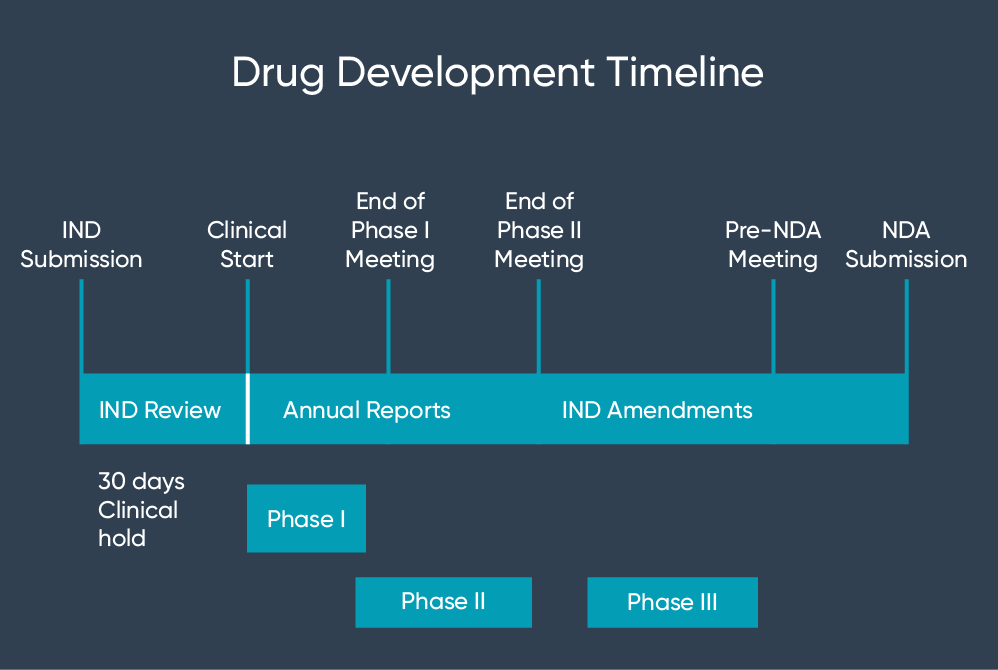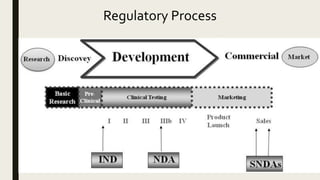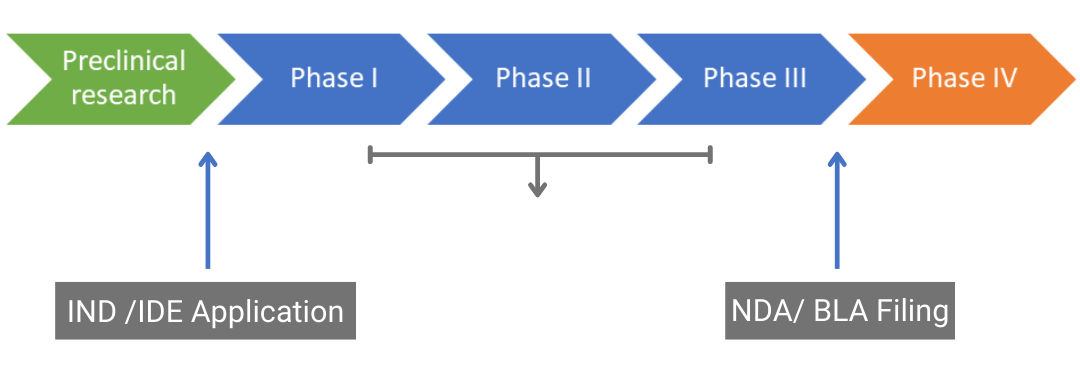
Drug approval pathway. IND: Investigational New Drug, IDE: Investigational Device Exemption, NDA: New Drug Application, BLA: Biologics License Application - ProRelix Research

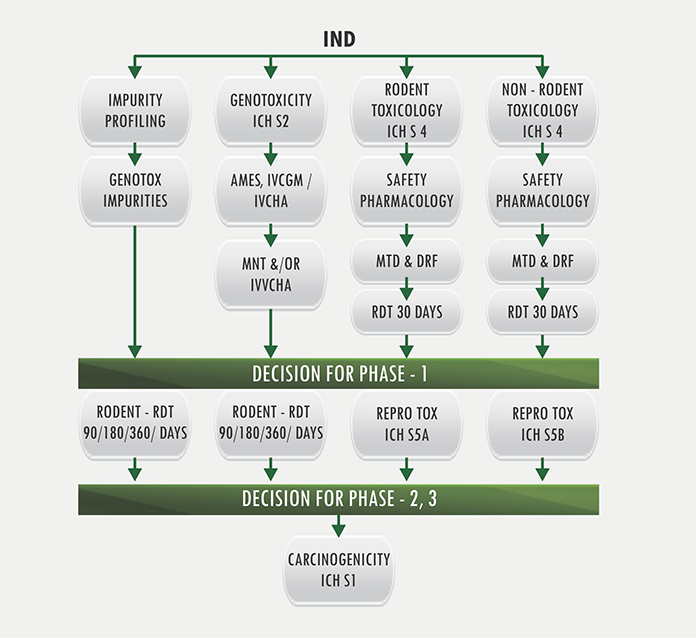
This flowchart represents the steps required for IND submission. Each... | Download Scientific Diagram